
 |
Temperature change in an insulated transport box |
Tim Padfield and Poul Klenz Larsen
The effectiveness of thermal insulation inside a transport case depends on the nature of the enclosed cargo. There must be material of high heat capacity within the insulated cavity to absorb the heat transmitted through the insulation. A picture on canvas, for example, has very little heat capacity and requires a case with a large surface area, so thermal insulation alone is not effective in holding a constant temperature around the picture.
The temperature can be stabilised better by putting the insulation outside the box, so that the heat capacity of the relatively massive box can be put to use. The insulation can be regarded as expendable and can be used to absorb impact by sharp objects.
Insulation within the box is often expected to satisfy the incompatible requirements for mechanical and thermal protection. It is much better to optimise the padding next to the object to reduce the acceleration caused by knocks and vibration, leaving thermal protection to a separate layer, outside the stiff container which provides mechanical protection.
A simple experimental cooling of a picture in an insulated case shows the typical rate of cooling, and the considerable effect of even the small heat capacity of a framed picture.
A wooden transport case with 100 mm internal insulation was used for the experiment. The case was cooled twice, once empty and a second time with a framed canvas picture inside it. The construction and scale of the case and the picture are shown in figure 1.

Figure 1. The painting, wrapped in polyethylene, in the insulated box. Two temperature data loggers are placed immediately above the picture.
The data loggers could only measure down to minus ten degrees, while the freezer continued down to -30°C. However, the trend at the beginning of the cooling period (figure 2) is enough to show what is happening.
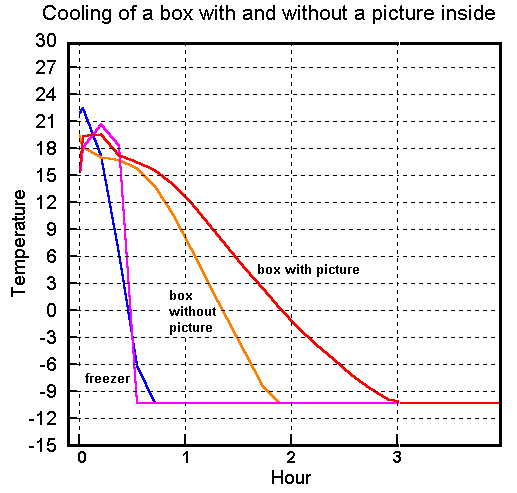
Figure 2. The rate of cooling of the air inside the box when it is placed in a freezer. The cooling of the freezer was not exactly the same in both experiments. The steeper cooling curve for the freezer is (by chance) when the box contained a picture, so the stabilising effect of the heat capacity of the picture is actually slightly greater than indicated by the slope of the curve. The flat line at -10oC is the limit of operation of the data loggers.
It is not possible to insulate thermally a nearly empty container that must endure a cold period of several hours. Hardly any heat needs to penetrate the insulation to make a large change in the temperature of the air within. Thermal stability can only be obtained when there is both thermal insulation and enclosed material that is able to absorb the heat that penetrates the insulation, without itself changing much in temperature.
The amount of heat which must be added to a material to cause a given temperature change is the heat capacity, which is described in the literature also as thermal capacity, specific heat and molar heat.
The heat capacity of the materials enclosed within the insulation depends on the nature of the material and the amount of it. Most materials have heat capacities in the range 500 to 2500 J/(K.kg). Water is the notable exception at about 4200. The unfamiliar units need not trouble you: think of this number as the heat energy needed to warm up a certain weight of material. Air has a heat capacity of 1000, but its low density, about one kilogram per cubic metre, means that the volume of air in the box doesn't absorb much heat. If the air space is filled with a solid block of polyethylene, for example, (density about 1000 kg/m3, thermal capacity 2300) the temperature will change two thousand times more slowly if heat leaks through the insulation at the same rate as with the air filled box.
Polyethylene has the highest thermal capacity of common box-building materials, with wood not far behind at about 1800. Aluminium has a thermal capacity of 920, which is better than steel at about 500.
For a given total weight of package an inner container of polyethylene would give a good performance.
A rational design for a transport case for lightweight objects of considerable extent, such as paintings, would therefore consist of the following elements, listed from the inside out:
1. The object
2. One layer of paper for humidity buffering
3. Polyethylene film, wrapped around the object but not sealed, so that pressure change during a flight does not deform the wrapper. A reasonably well wrapped object will buffer its own relative humidity, because the air exchange will be slow during the flight.
4. Energy absorbing buffers to hold the painting physically in place.
5. An inner case to provide rigidity and also to provide the main thermal buffering. (If this case is of wood it should be coated inside with aluminium foil to prevent outgassing of acid gases).
6. Thermal insulation, typically medium density expanded polystyrene. This functions also as an absorber of knocks by sharp objects.
7. An optional lightweight outer case to enclose the polystyrene, to allow attachment of handles and messages to take care in many languages. This will also provide some visual authority, so that baggage handlers may treat the packet with respect.
The standard wisdom on packaging paintings can be found in: Mecklenburg, Marion F. (ed.), Art in Transit, Studies in the Transport of Paintings, International Conference on the Packing and Transportation of Paintings, London 1991 National Gallery of Art, Washington, 376 p.
Acknowledgements: Thanks to Ingegerd Marxen for use of the freezer at the National Museum of Denmark and to painting conservator Barbara Berlowicz, whose correspondence with an airline prompted this experiment.
Copyright: Tim Padfield & Poul Klenz Larsen, July 2001

This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License.