 |
The absorption of water by materials |
In last month's article I described the diagram which defines the properties of damp air: the Mollier or Psychrometric Diagram. The reason for introducing that rather challenging mental gymnastic exercise is that water vapour in air is an important influence on museum objects, many of which contain water in a loosely bound form.
Wood is typical of the water-containing materials. Wood in furniture contains roughly ten percent water by weight. The exact water content depends on the temperature and humidity of the surrounding air during the previous few weeks. The water content matters to conservators because wood swells and shrinks at it absorbs and desorbs water. Water is also an accelerator of slow decomposition reactions.
Under what circumstances does wood exchange water with air?
If we take a five liter jar we can calculate, by reference to the Mollier diagram and the density of air, that the air within it, at room temperature, cannot contain more than about a tenth of a gram of water. If we now pour in a kilogram of wood chips the bottle will contain about 100 grams of water, almost entirely in the wood. We can therefore flush the jar quickly with dry air and wait to see what happens, knowing that the relative change of water content of the wood will be very small indeed.
In spite of the overwhelming reserve of water in the wood, the air in the flask does not become saturated with water but comes to equilibrium, after a short time, at about 50% relative humidity.
A graph of the equilibrium RH of the air in the bottle after equilibration with wood pre-adjusted to various water contents (EMC = Equilibrium moisture content) looks like this:
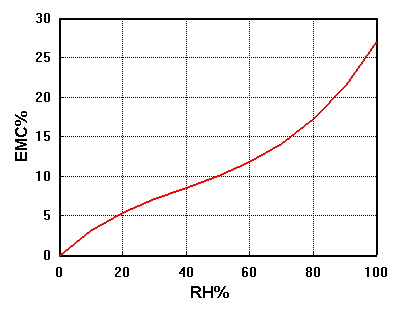
The exact curve depends a little on the species of tree, but the shape of the curve is always the same.
The curve is called the absorption isotherm, a jargon term which means that the curve joins a series of measurements made at a constant temperature, 15 degrees in this case.
We can complete the diagram by conducting the same series of measurements at various temperatures. The curves lie close together. Usually one can ignore the slight temperature dependence of the equilibrium, but there are circumstances where it is important: a point I will take up in a later article.
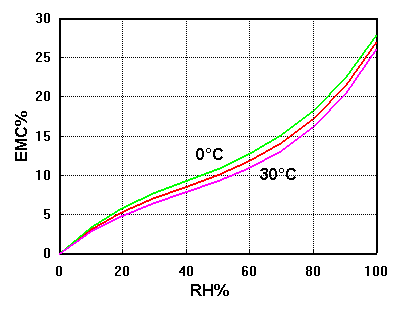
Many materials show similar behaviour: cotton, linen, wool, silk, silica gel and even concrete.
Because the equilibrium RH depends on the water content of the material, almost unaffected by temperature, conservators always refer to the water content of air in terms of RH. This is not strictly correct, because RH is just a ratio, which can only be converted into water vapour content if the temperature is also quoted. It doesn't usually matter but when it does, this casual simplification can cause a lot of misunderstanding.
Furniture is not usually displayed in glass bottles, even in museums. In the real world it is the air that controls the water content of the wood, because its low water vapour concentration is compensated by the continuous flow of fresh air past the object. Although the air is now controlling the water content of the wood, the results derived from the sealed jar experiments still apply. One must get used to thinking in both ways. Occasions when the sealed jar concept applies are quite numerous: packing for transport, freezing or heating in a sealed bag to kill bugs, flushing bagged objects with nitrogen or carbon dioxide to kill bugs, enclosure in a showcase.
At this point we have all the necessary tools to study the movement of water vapour between air and absorbent materials.

This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License.