 |
The isoperm and the isoburn |
Expressing the durability of materials as a lifetime is not very satisfactory. For us mortals the definition is appropriate: the heart is either pumping or stopped. Lifetime can be accurately defined, though seldom predicted. For a roll of film in an archive a small degree of degradation is enough to alter the spacing of the sprocket holes so the film tears in the projector and cannot be shown. If the same base material is used for a child's toy, much more degradation can happen before the toy becomes unrecognisable and ineffective in a museum display. It is better to quote a rate of reaction and leave it to the conservator to decide how much degradation corresponds to the end of useful life as an archive or museum object.
The array of pale curves in figure 1 shows combinations of temperature and relative humidity which give the same rate of hydrolysis. The curves are computed from the mass-action/Arrhenius equation derived on page 1. The rates are expressed relative to the rate at 20°C and 50% RH, and are valid only for a reaction with activation energy 100kJ/mol. Unlike the PI, there is no absolute value, expressed as degree of degradation per year, or lifetime in years.
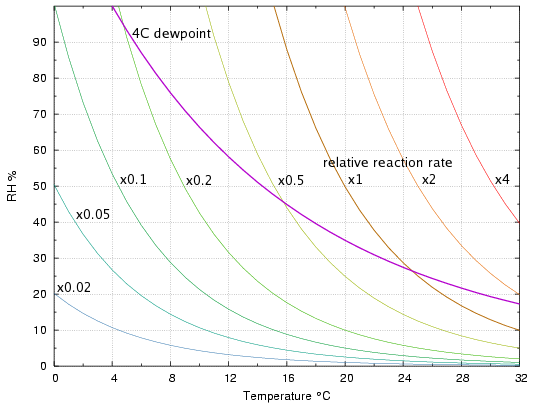
Figure 1. Lines of equal degradation rate, 'Isoburns', with the line of constant 4°C dewpoint superimposed.
As one slides a finger upwards along one of these curves, every combination of temperature and relative humidity will give the same degradation rate for materials susceptible to hydrolysis. Don Sebera (reference on page 1) gave the name 'Isoperms' to a set of curves which were similar in shape but defined lines of constant durability, which is the reciprocal of the rate. I show Sebera's figure below.
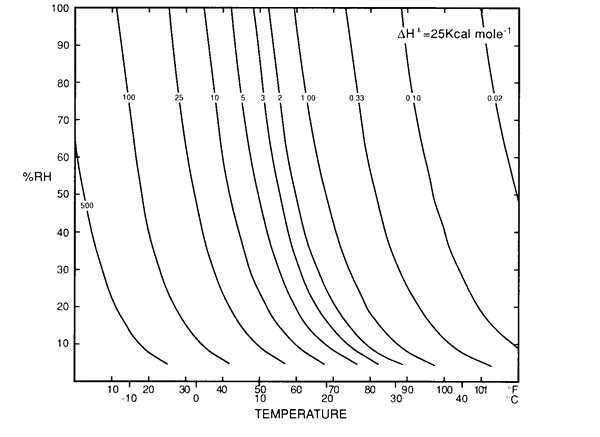
Figure 2. Sebera's isoperm diagram. It is similar to figure 1, but the lines are for constant durability rather than constant reaction rate.
Figure 1 also shows the line marking the 4°C dew point*. This particular dewpoint is shown because it is the practical limit for orthodox air conditioning with water cooling. If the air conditioning ducts can push out air with this dewpoint, one can choose to have a low temperature with a high RH, or any other combination along the path of the curve. The interesting observation is that it is better for the durability of hydrolysable materials if one selects a low temperature with a consequently high relative humidity, because the dewpoint line moves into a region of lower degradation rate as it ascends towards the high RH region. To put it another way, the temperature has a greater effect on the rate than the relative humidity, given a constant moisture content in the air.
'Conservation Heating' is the comforting term for gently heating a building to lower the relative humidity, but not too much. There is no adjustment of the water content of the air, which is very close to the prevailing outdoor value, so conservation heating is also constrained to a point somewhere on the line for the prevailing dewpoint. The same argument applies: it is better to have a high RH, with a correspondingly lower temperature. Conservation Heating is used widely in temperate climates to preserve buildings that are only intermittently used in winter and do not need to be continuously heated for human comfort. Figure 1 suggests that heating should be only just sufficient to inhibit biological growth. Growth also slows at low temperature as well as at low RH, so the optimum RH for preservation at low temperature is actually quite high, perhaps as high as 70% if the temperature is around 10°C. This needs very little heat, which is economical and has the important extra benefit that a small temperature difference between inside and outside minimises temperature gradients which are a potent cause of local condensation.
Returning to mechanical air conditioning, it is possible to dehumidify air below the 4 degree dewpoint mark, using coolers that defrost intermittently, or absorption dehumidifiers which use solid absorbers or salt solutions (be wary of the latter type). A lower dewpoint, which would move the curve on figure 1 to the left, is obviously good for reducing the hydrolysis rate. However, dehumidification is only economical in a tightly sealed building, a description which hardly applies to historic buildings deemed worthy of Conservation Heating. In a new museum store, however, dehumidification is certainly practical, and economical. The matter is discussed further in the second part of the article The Breath of Arrhenius and also in a paper from the ICOM-CC Dresden conference: Low energy climate control for museum stores.
The significance of dewpoint in this discussion is that mechanical air conditioning works by passing air over a surface so cool that water vapour condenses from the air stream. The air emerges cool and at 100% RH. Since there is for a given temperature a unique value for the saturation water content of air (actually space), the dewpoint is an alternative way of defining the water vapour content of air, even at higher temperatures where it is not saturated.
The air from the cooler is then warmed up, maintaining its water vapour content, as it was fixed at the moment it emerged from the cooler. The relative humidity and temperature of the air emerging into the air conditioned room is therefore at some point on the line of constant dewpoint.
In the case of Conservation Heating, there is no control of dewpoint, which is defined by the weather outside the building, but the indoor climate is still constrained to lie on the curve corresponding to this transient value of dewpoint. In reality, humidity buffering by the interior walls and furniture maintains a fading memory of the weather in the previous few hours. Powerful humidity buffering is not a feature of most historic buildings, though it can be used to advantage in well stuffed store rooms.
Tim Padfield, October 2004

This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License.