 |
Correlation without causality keeps alive the myth of ventilation as good |
It is depressing that the prestigious IIC conference on Preventive Conservation in Turin, September 2018, included an article and a poster that reinforce the myth that ventilation is good for preventing high RH and has preservative value, even in a constant RH environment.
Stephen Koob, in an otherwise lucid article on the preservation of
glass
("Caring for glass collections: the importance of
maintaining environmental controls" Studies in Conservation, 2018 vol
63 Supplement 1, 146 -150, DOI 10.1080/00393630.2018.1492252)
states: "Moving air creates lower pressure around an object. The lower
the pressure, the greater the volume of air and its ability to hold
moisture (1). As with fanning yourself on a hot day, the air movement
removes moisture from the object's surface."
The footnote states "This is Bernoulli's law, and can be further explained by the ideal gas law..."
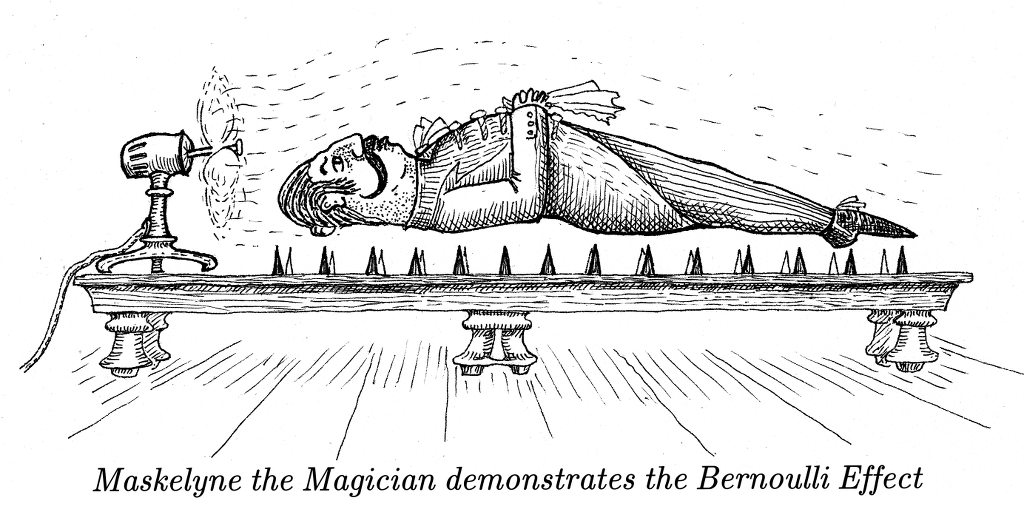
This paragraph, and its footnote, contain a sequence of errors of comprehension of atmospheric physics. Moving air does not create lower pressure - it is a pressure gradient that accelerates the air stream. A reduction of pressure occurs at a convex surface where a stream of air passes smoothly over it, like an aircraft wing. However, the static pressure, that is the pressure within the moving air mass, is the same as everywhere else. The air does not expand, since it is constrained by stagnant air around it. In any case, air does not hold water. The authors should have added John Dalton to their name-dropping note: in 1802 he asserted that in a mixture of gases, each will act entirely independent of the concentration of the others. Relative humidity equilibrium is unaffected by change of total gas pressure.
The fanning analogy is false, because people drink, to keep their skin surface moisture nearly always out of equilibrium with the ambient RH. Glass in an exhibit case does not drink. It has no continuous supply of water to evaporate, so it will rapidly achieve equilibrium with the RH in the enclosure.
The authors give no quantitative evidence for the phenomenon they describe, which is supposedly caused by a single fan in the bottom of a showcase which is not further described. If their assertion is correct, and of significant magnitude, The RH diminishes when the total pressure diminishes. It also increases when the pressure increases, as in concave areas of the objects. There would then be deliquescence of salts in concave areas, the liquid would migrate to convex areas and recrystallise there.
The reported RH within the case is compatible with the ambient RH at 40 - 45%, slightly lowered within the case by the heat from the fan. The claim that this arrangement "effectively prevents hydration occurring on the glasses" is just conservation by assertion.
Further on there is a paragraph about shapes that can trap moisture, with consequent high RH causing deterioration. The void would have to be totally sealed to prevent it gradually coming to equilibrium with the 40 - 45% RH prevailing for years in both gallery and storage at Corning.
Daguerrotypes are frequently observed with
clean glass outside and spots of liquid on the inside surface, sealed
within a display box that is only airtight with a half time to
equilibrium of at most a month or two
[http://www.conservationphysics.org/tis/tis.pdf
(figure 9) opens in a separate window]
and whose imperfection of
sealing is revealed by the rim of sulfide corrosion on the plate
itself. In the linked example, the mix of sodium, sulphate and formate
ions was still liquid in the 50% RH of the museum.
For permanent differences to persist between the surrounding RH and the equilibrium RH within an object, there has to be non-uniformity in temperature, or a continuous supply of water to the object. No source of water is identified in the Corning display and no temperature gradient is mentioned.
A poster by Morena Ferreira and six others is entitled "The influence of water activity and air movement in preventing mould in historic materials", Studies in Conservation, 2018 vol 63 Supplement 1, 348-350 doi 10.1080/00393630.2018.1471889
In this poster confusion comes early: "Microbiology studies and the food industry focus on water activity, (aw) instead of RH as one of the main parameters influencing mould growth."
However, water activity and RH are identical thermodynamic entities, with RH being the water activity multiplied by a hundred, for convenience in eliminating the decimal marker. Biologists use activity, meteorologists and conservators use RH.
What Ms Ferreira explains is that the RH in equilibrium with the surface of an object can be far from the RH at large in the ambient space. There is a state of disequilibrium through the boundary layer between moist solid and drier air. If one does nothing, the whole system will eventually settle down to a steady state with identical equilibrium RH within the pores of the object and in the ambient space. On the way towards this equilibrium a damp material can remain damp long enough for mould growth to begin before it dries to a safe equilibrium RH. Since the rate of drying depends on the air flow, so does the time available above a limiting RH for mould germination.
The experiment described in this poster is an investigation of the drying rates of various dampened materials subjected to different air flow over their surfaces. This process has been abundantly researched, since drying of cloth, paper, peas and dung are industrial processes which are energy intensive and benefit from exact research into drying rates. The diffusion rate of moisture through many materials has been measured and incorporated into computer models for moisture movement in building walls and through wooden sculptures.
One wonders why the experienced co-authors of this poster have repeated easily found experimental data, but more disturbing is the implied difference between water activity and RH, when there is actually no difference at all.
Both this and the Corning article assume a mysterious and continuous source of water, to maintain a permanent state of disequilibrium. In such a situation a vigorous air stream of low RH will certainly reduce the RH at the surface of the object. More important is to identify and reduce the source of the water. In historic structures the source is predominantly cold walls in spaces of uniform water vapour concentration, leading to locally high RH, which in turn produces a high equilibrium RH at the surface of the objects in the cold region. Direct injection of water, by rain penetration or rising damp is relatively rare, though often invoked, together with underground rivers, as a source of trouble.
The advocacy of air movement to counter mould growth, instead of cutting off the source of water, has a long history in conservation, even at the level of official guidance. The British Standard for Archives 5454 of 2000, now mercifully obsolete, made frequent advice to ventilate:
7.1 "Dampness and poor ventilation may encourage the growth of mould." and "...low temperatures with adequate air movement are preferable..."
7.4.1 "The air within the repository should not be stagnant. There should be sufficient air movement to avoid pockets of stagnant air."
7.4.2 "It is important that shelves be adequately ventilated to allow the free movement of air."
The standard does however, advocate boxing, which ensures stagnant air:
C.2.2 "Boxes and lids should be of acid-free board of 2 mm thickness."
I have never before on this website criticised named people (the standards are anonymous). I do so because there are two serious points to be made about ventilation mania and its careless transmission through the peer reviewed literature. The first is that it vastly increases the cost and complexity of storage and display, where the emphasis should instead be put on ensuring uniformity of temperature, so that localised high RH cannot arise. The second issue is that the widespread and persistent misunderstanding of basic scientific principles shames our supposedly science-based profession. The basics of thermodynamics and atmospheric physics seem not to have entered the syllabus of conservation training.
There are many articles on the internet about Bernoulli's equation
and its frequent misunderstanding and misuse. One of the more cogent
is by Weltner:
http://user.uni-frankfurt.de/~weltner/Misinterpretations%20of%20Bernoullis%20Law%202011%20internet.pdf
It
includes simple experiments to demonstrate air movement under pressure
gradients.

This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License.