 |
Humidity exchange with absorbent materials |
In an earlier page I described the nature of water
exchange between materials and air by showing what happens when scraps
of wood are bottled up with very little air. The air initially contains no
water vapour but it rapidly acquires a water vapour content which is dictated
by the water content of the wood.
 This is the reverse of the usual description of the phenomenon, where the material
is regarded as taking up, or losing, water to achieve a concentration dictated by the
relative humidity of the surrounding air.
This is the reverse of the usual description of the phenomenon, where the material
is regarded as taking up, or losing, water to achieve a concentration dictated by the
relative humidity of the surrounding air.
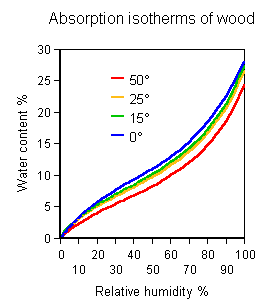
The graph of water content of wood against atmospheric relative humidity is the same, however one derives it (It is in fact easier to measure the family of absorption isotherms by measuring a set of RH values, at a series of temperatures, in a row of sealed containers, each containing a little air and a lot of wood which has previously been equilibrated at a defined water content).
I will now press the original wood-in-jar experiment a little further.
In the previous article I left the jar in an unheated store at 15 degrees. The RH in the jar was about 50% (I deal with the tricky matter of measuring the RH in a sealed container in a later article).
If the temperature is now slowly raised to 30 degrees, the relative humidity of the air in the jar will scarcely change, because the curves for the equilibrium distribution of water between air and wood are almost the same, regardless of temperature, when the atmospheric humidity is expressed as RH. On the diagram, the state of the system moves just a little, very nearly horizontally to the right, causing a slight rise in RH, about 3%.
The amount of water entering the air is a negligible portion of the water content
of the wood. This is why the equilibrium point moves horizontally across the graph:
the whole process occurs at nearly constant water content in the wood.
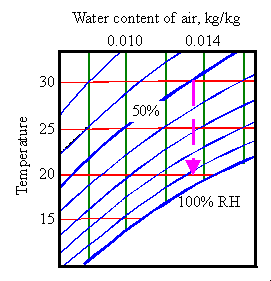 The
jar is now at 30 degrees and about 53% RH. Next, I quickly remove the
wood and immediately screw the lid firmly on again. I let the temperature
drop down to 20 degrees. From the Mollier diagram,
we find that the RH in the jar, unbuffered by wood, will now rise to more
than 90%. If the wood had still been there the RH would have fallen
slightly to about 50%.
The
jar is now at 30 degrees and about 53% RH. Next, I quickly remove the
wood and immediately screw the lid firmly on again. I let the temperature
drop down to 20 degrees. From the Mollier diagram,
we find that the RH in the jar, unbuffered by wood, will now rise to more
than 90%. If the wood had still been there the RH would have fallen
slightly to about 50%.
Conservators use a good deal of energy to persuade curators and engineers (and scientists), as well as each other, that 50% RH at all times is the ultimate good thing for museum objects.
If the original purpose of the jar, before I borrowed it for the experiment, was to store a museum object, it would provide a more constant RH if I returned it with the wood inside, to act as a stabiliser.
Unfortunately, wood provides not only water to the air in the jar but many other chemicals, such as acetic acid. We should therefore use another material, such as silica gel, or cotton, which gives a similar stabilisation of the climate, without contributing corrosive gases.
I will summarise the situation:
First: one asserts that relative humidity is an important variable to hold constant when the temperature changes, second: one notices that water absorbent materials tend to do this anyway, third: one surrounds valuable art with an abundance of such material.
In showcases or transport packages the buffering is usually done with paper, cellulosic cloth or silica gel, which comes in several varieties with different absorption isotherms.
In museum stores the objects will usually buffer each other.
A carved wood altarpiece has exactly the same physical properties
as a floorboard, assuming similar surface permeability. Storage space is so valuable in most
museums that there is no room for superfluous buffer material. Stuff the valuables
cosily close and they will stabilise each other. This separation of
physical properties from sentimental values troubles some conservators,
but they should think of the matter
like this: the object "sees" a particular climate. If one sneakily removes
the buffer and substitutes other museum objects which have the same moisture reserve,
the original object will notice no change in climate. The same argument applies
to each of the other objects, so there is no physical or ethical difference
between mutual climate buffering by crowded treasures and the same buffering
by elaborate confections of art-sorb in goretex.
There is one very important rule when using an abundance of buffer material to stabilise the climate around art treasures. The temperature of the buffer must be the same as that of the object. During rapid temperature change, when a packing case for example, descends from a cool stratospheric journey in an aircraft hold and is then left to bake on the tarmac of a tropical airport, the transient temperature difference between object and buffer can cause condensation of water on the object.
Humidity buffers are also used to stabilise against leakage of air of the wrong moisture content into the showcase, travel pack or storage room.

This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License.