
 |
Designing cold storage for film |
July 2009
This lecture was presented to the staff of the British Film Institute in Berkhamsted, 29 June 2009. There is a full screen lecture version (pdf)

1. There are three aspects to film storage which I will talk about. The first is the optimal environment.
2. If we accept that cold storage is necessary, we have to consider how to move stuff between the store and the human occupied environment.

3. When we have sorted out that detail, all that remains is to design and build the store.
I will take these issues in turn.
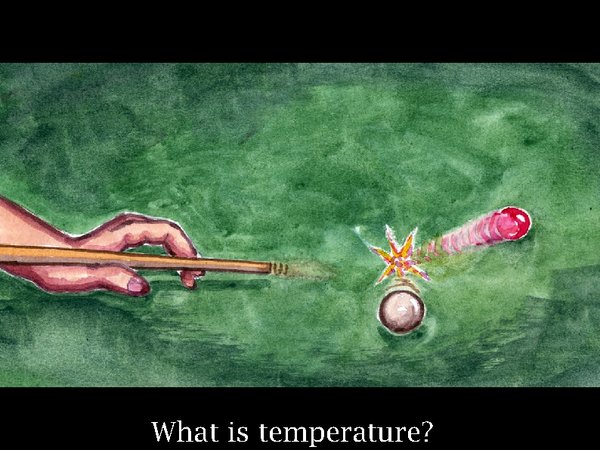
4. It's always a good idea to understand the environmental quantities under debate. Temperature is not so easy to define. It is, you may be surprised to know, proportional to the average energy of movement of molecules in a gas. I use the analogy of the snooker table. When you push one ball into another, energy is transferred unequally, it is possible to leave the first ball almost stationary, but you have imparted energy to the set of balls taken as a whole.
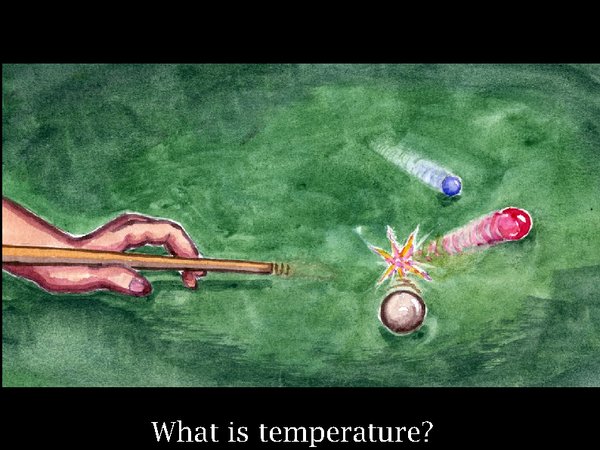
5. In the next step in the analogy imagine all the tournament players simultaneously introducing energy, known as heat, from the edge of the table, so there is a chaos of bouncing and colliding balls, moving typically at 400 m/s in the case of air molecules.
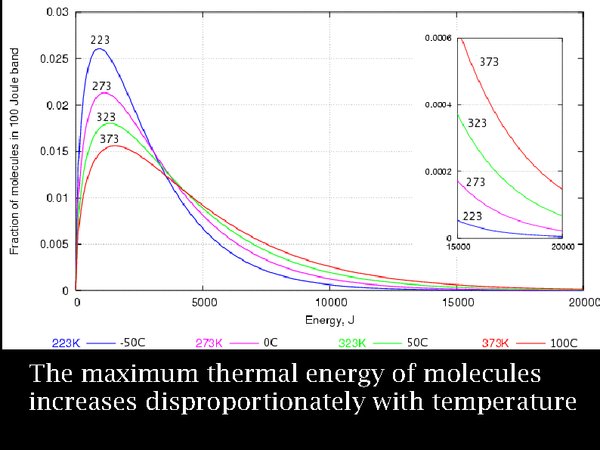
6. Over a century ago, Maxwell and Boltzmann, quantified this process. This diagram shows the energy distribution of large numbers of atoms at equally spaced temperatures, expressed in degrees Kelvin. Notice that the most abundant particle energy, the peak of the curves, increases steadily but not dramatically with temperature. The important point to note is that at the highest temperature, 100°C, the number of high energy particles is notably enhanced. The last part of the diagram is enlarged in the inset. The Swedish chemist, Svante Arrhenius, explained the rapidly increasing reaction rate of esters, such as cellulose esters, with temperature, as due to the small number of very high energy molecules, so reaction rate was much more than proportional to temperature - it was exponentially increasing.
7. Astonishingly, this apparently naive treatment of colliding molecules accounts for the observed reaction rates of very many chemical processes, not just in gases, and has been amply confirmed over the intervening century.
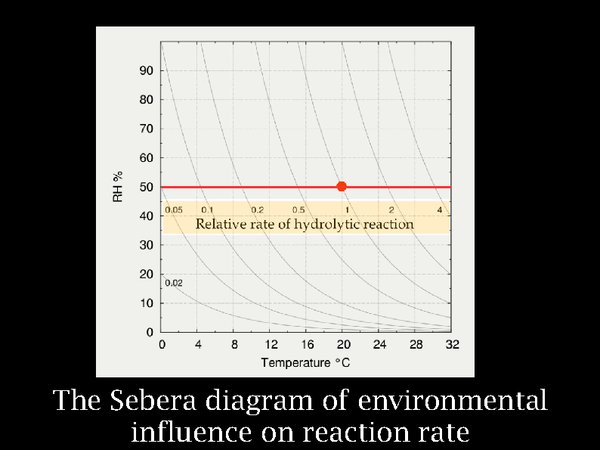
The insight of Arrhenius was presented as a diagram by the chemist at the US National Archives, Don Sebera. Because most decomposition reactions of polymers, such as cellulose acetate and gelatin, involve addition of water with breaking of molecular bonds, he added the dimension of relative humidity to the Arrhenius concept. But let us first consider the horizontal red line, representing variation in temperature at constant RH. The red spot marks room conditions. If one lowers the temperature to 15°C, the reaction rate has already halved, at zero C it is a twentieth of the room temperature rate. It is difficult to prove this with relatively stable polymers, so our evidence comes from extrapolation of reactions studied at several quite high temperatures.
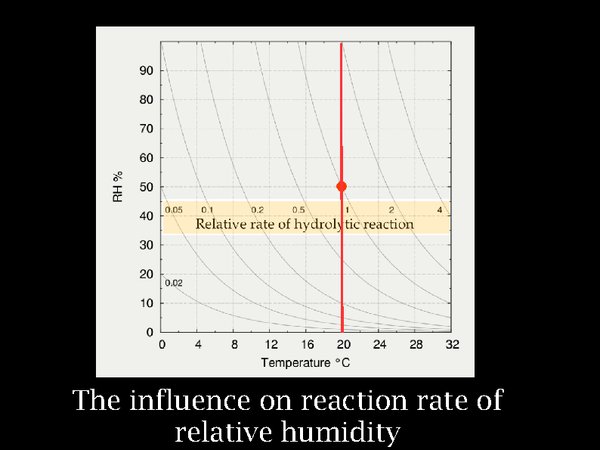
8. The influence on reaction rate of relative humidity, which can be considered to represent the ability of water to enter into chemical reactions, is considered to be proportional, rather than exponential. This results in reaction rates that bunch up at very low RH. One has to go from 50% to 90% RH to double the reaction rate, but from 50% to 5% the reaction rate reduces to one tenth.
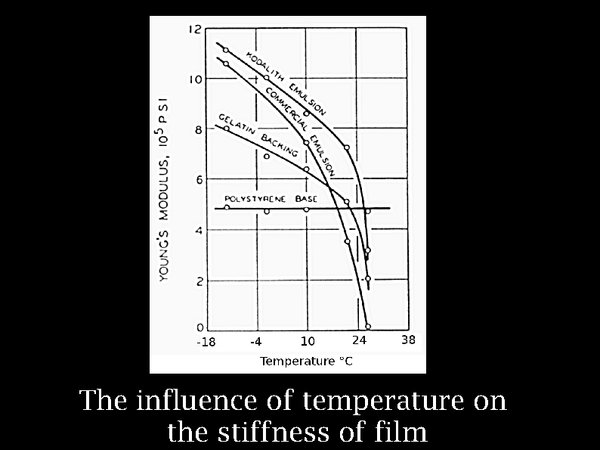
9. It seems that cool and dry is the way to go, but let us pause to consider the side effects. Chemically, and from experience from archaeology, there is abundant evidence for the preservative effect of cold or dry conditions. But laminated materials such as film risk breaking mechanically when taken far from the condition of their manufacture. This graph shows the increasing stiffness of gelatin, as a free hanging layer, detached from the film base. The increase of stiffness, here plotted as Young's modulus, is significant, but not extreme. Furthermore, the thermal expansion coefficients of the components of film are quite similar, so no serious stresses arise on cooling.
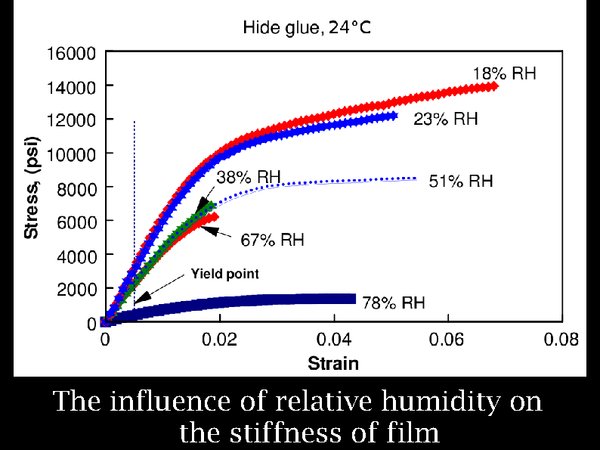
10. Turning to the effect of RH, low RH also stiffens polymers. here are the data for animal glue. However, even at 18% RH, the increased stiffness, indicated in this graph by the steepness of the curve, is not extreme.
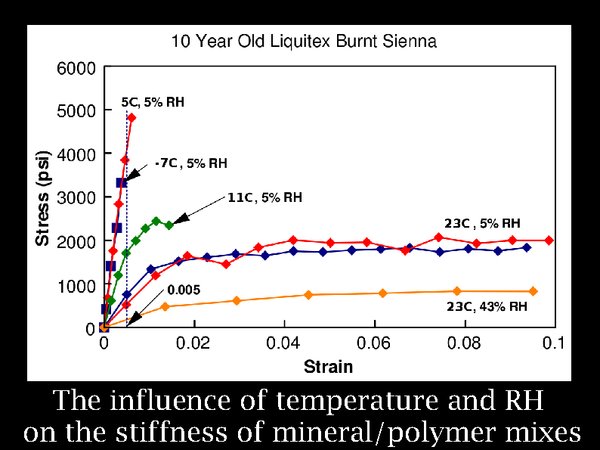
11. Many other laminated materials find their way into archives, often with heavily mineral filled layers. The effect of low RH and temperature is always to increase stiffness, and brittleness. The limit for responsible storage is set by experiment and experience. That has resulted in a tendency to accept a very low temperature but a relatively high RH, currently about 35%.
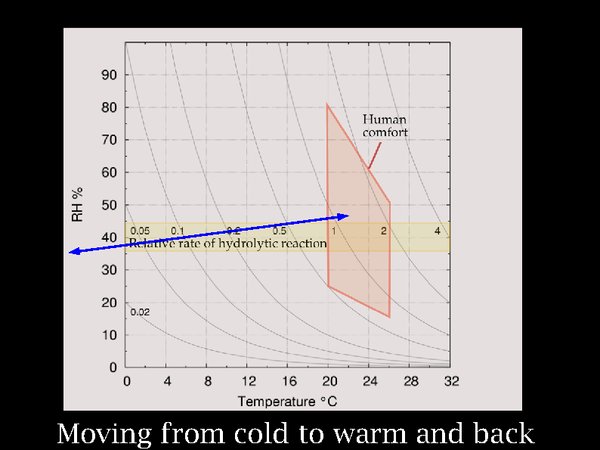
12. I move on now to the process of moving film between a cold store and a warm room. One can see from the nearly horizontal arrow in the diagram that the change in RH is not large but the change in temperature is worryingly extreme.
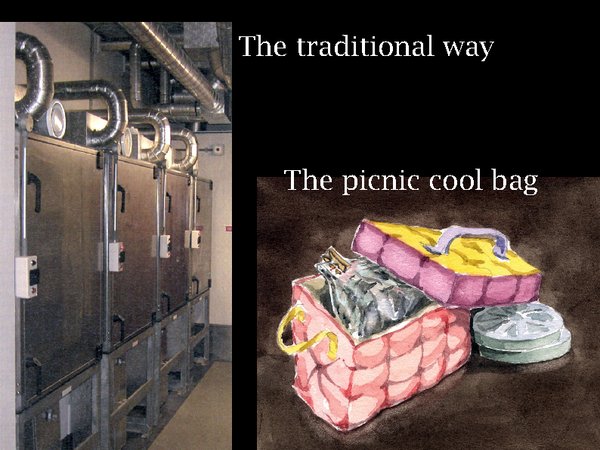
13. Many institutions have a series of intermediate air conditioned chambers, seen on the left. Since the ultimate purpose of this lecture is to explore low energy solutions to archival storage, I present on the right what I claim is a better solution, certainly it is a lot simpler. Just put the film into a fairly close fitting polyethylene bag, put it in a picnic food container (without frozen cooling blocks) and park it for a few hours in the projector room, or in the cold store on the way back.
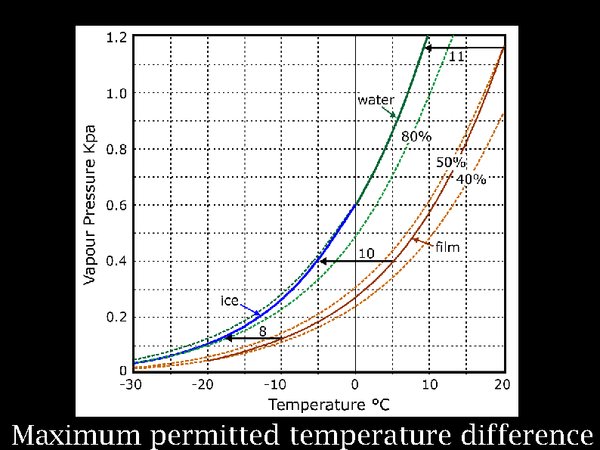
14. The reason for the line of conditioning chambers is fear of condensation on the film if it is brought straight into a climate congenial to humans. Logically, one should first worry about the risk of putting the film into the cold store before worrying about taking it out again. This rather complicated diagram describes this process. It shows the water vapour pressure (which is an indirect way of indicating the concentration of water vapour in the atmosphere) in contact with film at a moderate 50% RH. That is the heavy brown curve. As the temperature falls, the equilibrium RH within the roll of film falls very slightly. This brown line is far from the blue green line representing the saturation vapour pressure. So there seems to be no problem, indeed I don't remember reading any instructions to take care when putting film into a cold store. But think of this: when the film container suddenly finds itself in the cold, it cools down first, while the film roll, which has a considerable heat capacity, cools much more slowly. The horizontal lines indicate successive conditions of the film can and the film, as they both cool down. The arrowed end indicates the can temperature at which moisture released from the film will condense on the inside surface of the can.
The gap between the brown and the green/blue line is fairly constant regardless of the instantaneous temperature, at about 10 degrees. So this is the permissible temperature difference anywhere within the assembly of the film in its container.
In practice, changing the temperature from +20°C to -20°C slowly over three hours, will never cause the gradient in temperature to come near this difference. So I assert that the film, in an airtight wrapper, in a picnic food insulated bag, will be safe. If you doubt this, measure the temperature within some test packages.

15. This is a picture through a transparent window made in a metal can which was dumped into a domestic freezer at about -18°C. After a few minutes, ice crystals appeared on the inside of the window, caused by condensation of water distilling from the still warm film roll.

16. Now we have persuaded ourselves that cool is good, and movement of goods between temperatures needs only simple precautions and patience, we can move on to the building. This is the recently opened cool store for the world's seeds, in Svalbard. It opened last year with a promise of thousand year durability, but it has since been repaired because the entrance shaft failed in the permafrost. So we have to think carefully about the construction of cold stores.
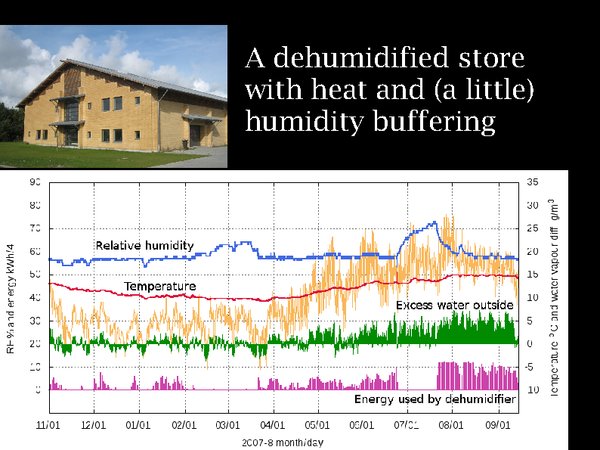
17. Before plunging into the cold, I want to show you a moderately cool store, in Ribe, southern Denmark, a region with a climate not unlike central England. It is not cooled but it is dehumidified. Look first at the temperature over a year. It is very stable, buffered by the massive walls of the building and by the uninsulated floor, which connects it to the approximately 10°C temperature at 3 metres depth in Denmark. When the dehumidifier fails, however, the picture is not so good. In winter, the failure results in a gentle rise in RH but in summer the RH rises to a dangerous value within two weeks.
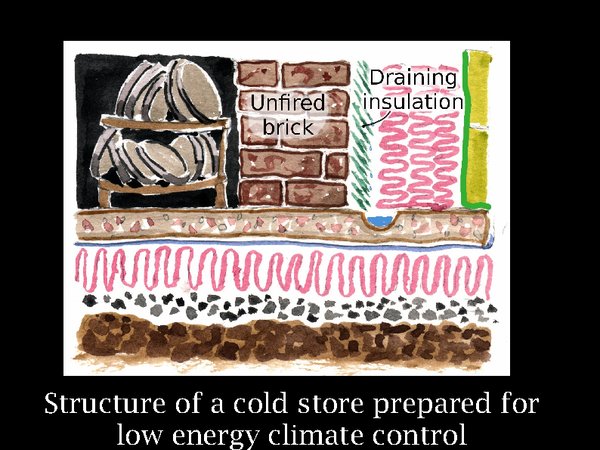
18. Using the Ribe store as a built example, and Svalbard as a warning against hubris, let us fantasise over how a cold store could be designed to be resistant to power cuts, social disorder and plain incompetence, in the far future of course. Firstly, we need good thermal buffering. That is the massive brick wall. Why I use unfired brick will come later, but we remark already that it is energy efficient, needing no kiln firing. Outside this is the insulation. According to thermal dogma, it is in the wrong place, it should be on the inside of the wall. However, the wall cannot then buffer the temperature, so it has to be outside. This brings the certainty of condensation as warm summer air diffuses into the structure. We react to this by putting in an air barrier, the green vertical stripe. But archives are not built like aircraft, so we must expect some leakage. So the inner layer of insulation is designed to shed condensed water into a drain. This will only happen so long as the insulation is above zero at that point. The design brief is -5°C, which is a bit too cold for this design, we have to hope that condensation will be slight. This depends on the competence of the supervising architect in my experience. The floor, unlike at Ribe, is insulated, because the store temperature is 15°C below the annual average in middle England.
Using solar cooling will, with present technology, require solar panels about four times the footprint of the building. However, if we rely on conventional power sources we can be sure that loss of power will not be catastrophic. The room will slowly warm and the RH will be buffered by the unfired brick.
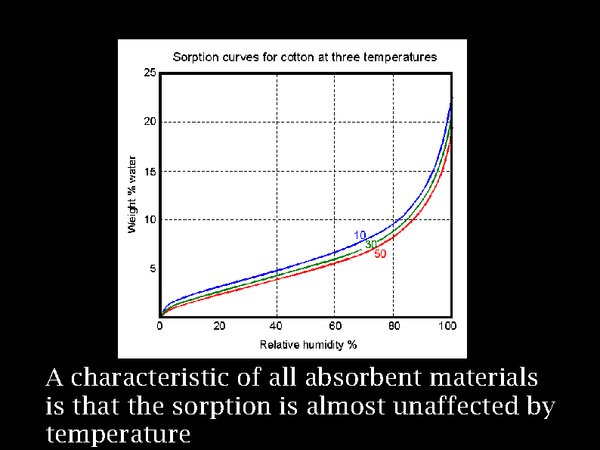
19. We need to take care of the relative humidity, because biological attack is a more immediate threat than the mildly accelerated decomposition caused by warming to a temperate climate.
One of the curiosities about absorbent materials is that their water sorption depends on the prevailing RH, with hardly any influence of temperature. This is shown here for cotton, but all materials have similar properties.
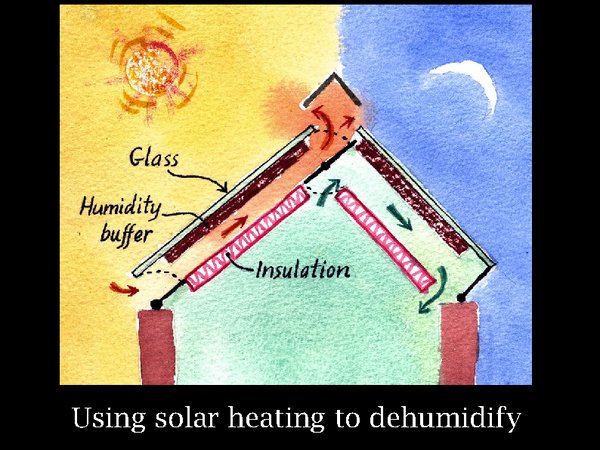
20. Here is how to combine this characteristic with solar power. On the left, solar energy on a warm day is heating a humidity buffer material, silica gel or even paper, while outside air is allowed to convect past it. The buffer loses water vapour to the air stream and will, on a typical summer day, reach an equilibrium RH within its pores around 10%. Move your glance now to the right side representing a cloudless night. The absorber has been allowed to cool by radiation to the night sky, isolated from any air flow. Then the air channel is opened to allow inside air to circulate past the absorber, which is at equilibrium with just below 10% RH. So the air is dried. It is also warmed a bit, so some detailed design is needed to ensure that the elegant free dehumidification does not demand more power from the cooling system than it saves. This is a diagram of the principle, there are many variations possible to reach a practical device. The total area of solar receiver would be about a quarter of the roof. That is if the store is reasonably airtight.

21. Which brings me hard up against a serious problem. Film stinks, because it slowly releases acetic acid, and other volatile substances. Here is an object made of nitrocellulose, a sculpture by Antoine Pevsner. Note the beads of liquid on the right. They are calcium nitrate solution. A consequence of the reaction between decomposing cellulose nitrate with the white filler, calcium carbonate.
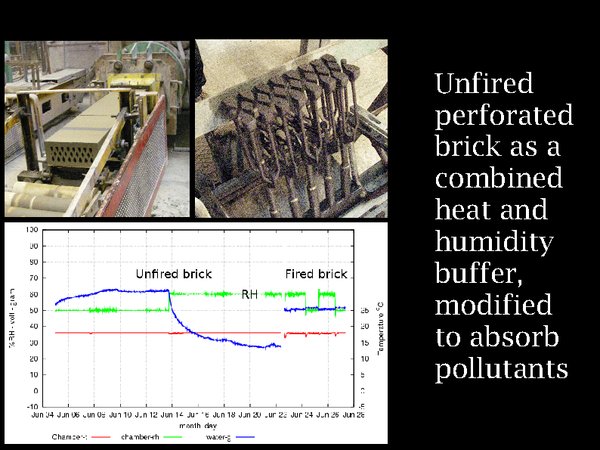
22. Again, unfired brick comes to the rescue, maybe. We have already learned that it is a heat buffer. Perforated clay brick is a commercially available material, seen emerging from the extruder on the top left, with the perforation form shown on the right. It has an enormous internal area for sorbing volatile chemicals. If it comes from a calcareous clay, as here in north Sealand near Copenhagen, it will react with acid gases. Its humidity buffer capacity is shown in the graph, hot from the experimental apparatus in Denmark's Technical University. A change of RH from 50% to 60% has resulted in a 30 gram absorption of water from 0.2 sq.m of unfired brick (shown in the graph inverted). To the right is the corresponding experiment with fired brick, showing barely 2 grams of exchanged water vapour.

23. Unfired clay brick is reckoned to be the world's most used building material, but it has been totally forgotten by the clever people in the advanced nations. One does not need to be a sentimental medievalist to appreciate that earth has qualities of humidity levelling which are entirely lacking in modern building materials.

24. This lecture draws on the research of many people. I particularly thank my colleagues in the National Museum of Denmark, Poul Klenz Larsen, Lars Aasbjerg Jensen and Morten Ryhl-Svendsen. Illustrations are adapted from articles by J. Calhoun, Don Sebera and Marion Mecklenburg
This lecture was presented to the staff of the British Film Institute in Berkhamsted, 29 June 2009.
Creative commons licence: free to use for any non-commercial purpose, but the author must be acknowledged and no changes may be made.

This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License.