 |
Film cooler for small collections |
by Tim Padfield and Jesper Stub Johnsen
This is the internet version of a poster presented at the 1994 meeting 'Preventive Conservation' of the International Institute for Conservation, in Ottawa, Canada. It was published in: Jesper Stub Johnsen. 'Archival survival of photographs' Dr.Phil thesis, Göteborg University Institute of Conservation, 1997. ISBN 9173463183 9789173463188
Our invention is an ordinary domestic freezer fitted with an inner insulated box, which is electrically warmed to about eight degrees above the temperature in the freezer. This automatically holds the relative humidity in the inner box near 50%.
Large museums and archives have walk-in cold stores which are air conditioned in a conventional way. Small collections are put in ordinary refrigerators or freezers. The objects must be sealed in airtight bags to prevent water absorption from the cold humid air.
We present a middle way for small collections: a freezer which holds a moderate relative humidity and therefore does not require that the objects be in sealed containers.
We use the word 'freezer' to describe the whiteware widely used to store frozen food, which is indeed full of ice. Film and other articles in equilibrium with a moderate relative humidity (RH) do not freeze, in the sense that ice forms within them. The safety of keeping film well below zero degrees has been abundantly demonstrated (see references below). For such materials of moderate water content, the passage through zero degrees has no special significance.
The space within a freezer is always nearly saturated with water vapour. This is because the room air that slowly drifts into the freezer nearly always carries in more water vapour than can exist in the cold interior of the freezer, so ice builds up slowly on the inside of the freezer while the space remains close to 100% RH. It is true that in very cold weather even the room air will not reach saturation as it infiltrates the freezer. However, the layer of ice clinging to the walls of the freezer will maintain saturation of the interior space.
The picture below shows a section through a freezer which contains an inner container which is warmed. Room air moves very slowly into the freezer while the trapped air circulates by convection through the warmed inner container. The warm air, without further access to water vapour, falls in relative humidity as it enters the inner chamber. The stored materials are contained within the inner chamber, in a moderate RH but still very cool.

Let us put some quantities to this brief description. Suppose that the freezer is at -20°C, the (saturation) water vapour pressure will be 560 pascal.
Now suppose that the inner box is warmed to -12°C. The temperature difference will cause a slow convection of air from the bottom of the freezer, through a small hole in the bottom of the box, up through the box and out past the loose fitting lid.
At -12°C the saturation vapour pressure of water is 1120 pascal, twice the value at -20°. The air that has convected into the box has no way of increasing its water content to this value. It will retain its original water vapour pressure. The relative humidity is the ratio of the actual vapour pressure to the saturation vapour pressure, in this case 50%.
In use, the inner box will contain materials, such as film, which can exchange water vapour with the air. However, if the film is preconditioned to about 50% RH, the air passing through the box will already be in equilibrium with it and there will be no exchange of water vapour.
This description is simplified, because the freezer does not maintain a constant temperature. It cycles over a range of about five degrees, because the compressor of the cooling system does not like to be restarted frequently against the coolant gas pressure. We return to this detail later.
The figure above shows an idealised vertical section through the freezer and the inner box. There are many variations on how to build such a system.
Our solution is sketched in the next diagram. The outer container is a standard chest freezer. It is not modified in any way. The power and sensor leads to the inner box are drawn through the drain hole in the bottom.
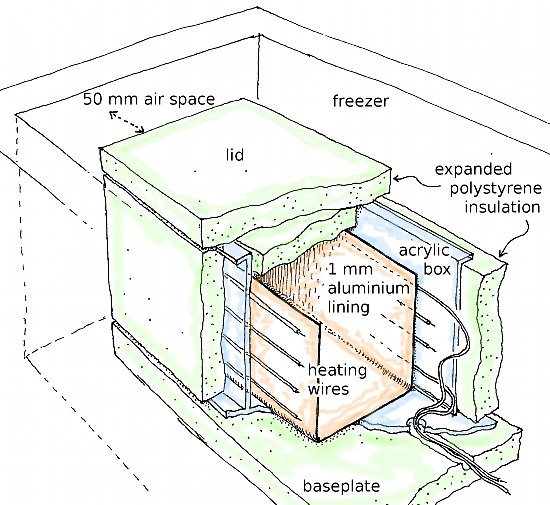
The inner box is made of acrylic sheet, insulated with slabs of polystyrene foam, 50 mm thick. It has a loose fitting lid of polystyrene slabs. The heating element is bonded to an aluminium sheet which covers the long sides and base of the acrylic box. The insulated box is separated from the freezer walls and floor by a 30 mm gap. This allows free air circulation and permits a moderate accumulation of ice in the freezer. This is an important regulatory component, because it ensures buffering of the air to 100% RH in cold weather, when the air drifting into the freezer has very little moisture.
Heating the inner box to -12°C presents few difficulties. The box is in the uniform environment of the freezer and has a constant rate of heat loss. It requires therefore a constant supply of energy. This is provided by a constant current through about 10 m of resistance wire (0.3 mm constantan wire insulated with PTFE) attached behind the inner metal lining of the box. The power supply is about 12 V, supplied directly from the secondary winding of a transformer, which is tapped to allow coarse adjustment of the voltage. Fine power control is by adjusting the active length of the heating wire.
Once the box is calibrated there is no need for further adjustment. The only active control is the freezer's own thermostat, which will be reliable because the freezer is a widely sold consumer product and has to survive in a competitive market. The only maintenance required is occasional defrosting (in the warm season).
The heat loss from the insulated box, 1.0 x 0.4 x 0.4 m, at eight degrees above its surroundings, is about 15 W. A freezer to hold this box typically consumes about 60 W, without the warm box inside it. The total consumption of freezer and box will therefore be roughly 100 W. In general one can reckon that the running cost will be about double that of a freezer in normal use.
The simplicity of the power supply ensures reliable operation. Nevertheless, since only an irreplacable collection would justify such an elegant climate control, a minimum amount of electronics is advisable to protect against damage resulting from failure of the various components. It is also a good idea to show the actual temperatures in the freezer and the box, to reassure the owner that all is well.
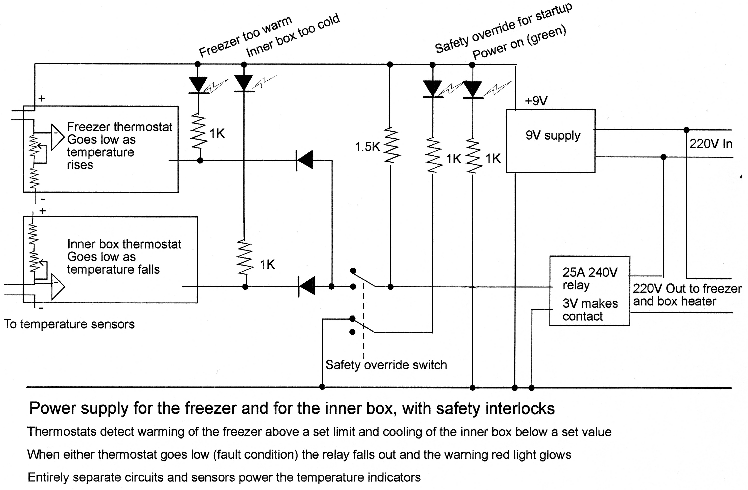
The inner box has temperature sensors (thermistors) mounted on its inside and outside surfaces. These are connected to an electronics box that displays the two temperatures and also contains safety interlocks that cut power to the freezer if the supply to the inner box fails and cuts power to the inner box if the freezer fails (see the appendix). Connection to an electronic surveillance system is also possible.
An advantage of this design is that there is no need to panic if the power fails. As the temperature gradient falls to nothing the convective air movement will stop. There is enormous buffer capacity in the stored objects. The store can stand without power for many months without any danger to the contents. The ice will melt and drain out, room air will infiltrate the freezer and the inner box will have its climate kept moderate by the stored objects.
There is no need for elaborate enclosure of the objects within the inner box. This increases the risk of damaging condensation when one is brought out of the box. The object must be slipped into an insulating airtight bag within the pool of cold air in the freezer before removing it into the room. The same precaution is necessary when replacing the object, as described in the article about tranferring film to and from a cold store. This article describes the subtle danger from distillation of water from one part to another of an object, caused by different rates of cooling when the object is replaced in the cold store.
One can expect a degree or two of variation within the inner box. However, the five degree variation within the freezer is entirely suppressed by the insulation and the thermal mass of the stored objects. The graph below shows a typical record of the cold store in use, with eight large film cans within. The higher temperature in the centre of the mass is strange, perhaps due to uneven heat distribution within the aluminium plate used to spread the heat from the resistance wire.
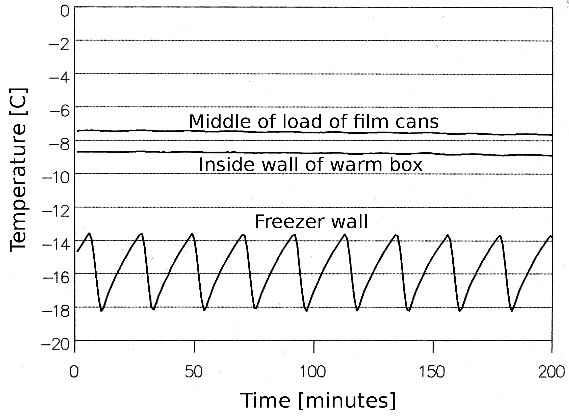
The exact temperature excess within the inner box is not important. The essential thing is that there should be no great relative humidity shock when the stored object is finally unwrapped in the room where it is to be displayed. A five degree temperature excess over the average in the freezer will give an RH below 66%.
One can imagine many other ways of generating heat for the inner container, waste heat from the cooling system for example. However, the beauty of the storage solution described here is that it is independent of the freezer technology. The inner box and its simple, constant power energy supply are independent of the make of freezer and its exact size. One could use a vertical format freezer, noting that these tend to have a lower internal RH, so a smaller temperature excess is necessary, 5 degrees being quite sufficient. However, it is difficult to remove objects from a vertical style freezer without risking condensation.
Measuring RH at low temperature is not easy. There is not much water vapour around and dropping in self contained data loggers may mislead, because of inadequate temperature compensation of both the sensor and the processing electronics. Since we expect the RH to be very slowly changing, we can lay in the box a strip of gelatin, about 1 mm thick (don't use film - its gelatin layer is too thin), previously conditioned at room temperature to 50% RH, say, and weighed. Remove the strip after two weeks, putting it swiftly into a tight fitting (weighed) airtight bag within the freezer. Let it warm in the bag to room temperature and weigh it again. You can use the published sorption curve of gelatin to estimate the RH within the container. Remember that conservators use RH constancy as a shorthand for what they really want, which is constant water content in the object. So if the gelatin hasn't changed weight much, the RH must be right, whatever it is. The slow rate of sorption of water at low temperature has been measured by Adelstein et al (see references)
The cold store will operate safely if the constant power supply to the inner box is taken after the freezer switch, so both stop working together. This may invalidate the freezer guarantee, so the next best solution is to run the heater from the same wall socket as the freezer. If the freezer fails without interrupting the power supply, the inner box will rise to five or six degrees above ambient, which will not be immediately disastrous. If the inner box fails without the power failing, through a break in the heating element for example, the RH will gradually rise to over 90%. This will be buffered by the stored materials and will be slowed by the low water vapour concentration at the low temperature. You probably have several weeks to discover the fault before damage is inflicted on the objects. If a more active monitoring is thought necessary, the diagram below shows the operational amplifiers used to deactivate the freezer when either the freezer or the warmed box fail, as indicated by an out of range temperature. This is just intended as a guide to the technician rather than as an exact circuit. We must report that this safety circuit was the first thing to fail when we set the prototype cold store in operation. We recommend the minimalist solution of just displaying the temperatures inside and outside the inner box. A type T thermocouple is suitable. Both thermocouple wire and display instruments are cheap and reliable from omega.com. See the datalogging tutorial for more information.
Development of this prototype micro cold store was financed by the National Museum of Denmark. A search of the scientific and patent literature up to 1994 has not revealed an earlier description of this simple way of moderating the humidity within a cold store for museum and archive use. We assert our right to be regarded as the inventors of this device.
P.Z. ADELSTEIN, J.-L. BIGOURDAN, & J.M. REILLY. 'MOISTURE RELATIONSHIPS OF PHOTOGRAPHIC FILM' Journal of the American Institute for Conservation 1997, Volume 36, Number 3, pp. 193 to 206. http://cool.conservation-us.org/coolaic/jaic/articles/jaic36-03-002.html This article describes the slow sorption of water into film at low temperature, which reduces the danger from temporary failure of RH control in a cold store.
J.M.Calhoun. 'The physical properties and dimensional behaviour of motion picture film', Journal of the society of motion picture engineers, vol 43 October 1944 pp 227-266. This paper is interesting in its consideration of the (reversible) embrittlement of film at low temperature. He writes that film at -32°C and 60% RH has the same brittleness as film at 21°C and 14% RH. Nevertheless he reports that film projection in the arctic is usually trouble free. One can assume that gentle handling of film rolls or flat film while it is cold is not risky, but flexing the film is inadvisable.
J.M.Calhoun. 'Cold storage of photographic film' Photographic Society of America Journal section B, vol 18B )October 1952. Demonstrates the safety of moving film in and out of cold storage. Note that the discussion rejecting the risk of condensation on putting film into cold storage is wrong, since it takes no account of the transient temperature gradient within the container.
D.F.Kopperl and C.C.Bard. 'Freeze/thaw cycling of motion-picture films' SMPTE Journal (Society of motion picture and television engineers), August 1985, pp 826-7. A thorough study which shows the safety of repeated cooling and warming of film.

This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License.